Biosimilars and Biologics Market Growth Driven by Patent Expiries & Innovation | DataM Intelligence
Driven by aging populations and patent cliffs, the biosimilars & biologics market will grow from ~$531B in 2024 to nearly $1.8T by 2033, says DataM Intelligence
DELAWARE, DE, UNITED STATES, July 24, 2025 /EINPresswire.com/ -- Biosimilars and Biologics Market Overview
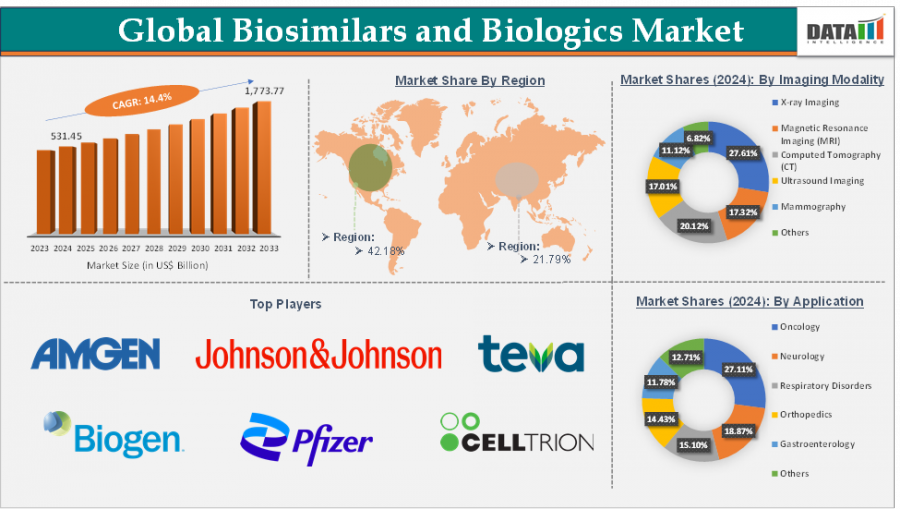
Biologics: It is a complex therapies derived from living organisms—are increasingly central to treating cancer, autoimmune diseases, insulin-dependent diabetes, and other chronic conditions. According to DataM Intelligence analysis, the combined global market for biosimilars and biologics reached approximately USD 531.45 billion in 2024, and is projected to grow to USD 1,773.77 billion by 2033, reflecting a robust CAGR of 14.4%.
Biosimilars, highly similar and clinically comparable versions of reference biologics, are enabling more affordable access to life-saving therapies—especially as major biologics face patent expiration. According to DataM Intelligence, the global biosimilars market is expected to reach USD 171.79 billion by 2033, growing at a striking 25.5% CAGR.
Download exclusive insights with our detailed sample report (Corporate Email ID gets priority access): https://www.datamintelligence.com/download-sample/biosimilars-and-biologics-market
Biosimilars and Biologics Market Growth Drivers
DataM Intelligence identifies several influential forces accelerating this market:
• Patent Expiration of Biologic Blockbusters: As originator biologics lose exclusivity, biosimilars capitalize on growing opportunity windows.
• Aging Populations & Chronic Disease Burden: Rising incidences of cancer, diabetes, and autoimmune disorders increase demand for both innovator biologics and cost-effective biosimilars.
• Healthcare Cost Containment: Payers and hospitals are under pressure to reduce drug expenditure; biosimilars offer 15–40% price savings versus brand biologics.
• Regulatory Clarity: FDA and EMA biosimilar pathways, including interchangeability designations, expedite market entry and clinician confidence.
• Physician & Patient Acceptance: Growing real-world data and education efforts have enhanced trust and adoption rates.
Regional Insights in Biosimilars and Biologics Market
North America
The United States leads globally in biosimilar and biologics consumption, supported by streamlined regulatory pathways and payer mandates. By 2025, major payers like UnitedHealth, Cigna, and CVS are prioritizing biosimilars like Amjevita for Humira, reinforcing adoption.
Europe
Europe holds significant market share in biologics and biosimilars uptake, supported by EMA approval systems and aggressive pricing competition.
Asia-Pacific & Japan
Asia-Pacific—particularly Japan—is emerging as a fast-growing region. In early 2025, Biocon Biologics gained PMDA approval in Japan for its ustekinumab biosimilar (Imuldosa) to treat psoriasis and psoriatic arthritis, marking increased biosimilar access in the Japanese market. India’s biosimilar sector is also gearing up with regulatory reforms, aiming to drive exports to $1 trillion by 2030.
Industry Momentum: Collaborations & Key Events
• Fresenius Kabi’s Otulfi Launch (March 2025): The ustekinumab biosimilar to Stelara received FDA approval and is now available in the U.S. for psoriasis and inflammatory bowel disease indications.
• Yesafili & Biocon-Regeneron Settlement (April 2025): U.S. launch cleared for the aflibercept biosimilar Yesafili (Eylea reference) following a licensing agreement—expected in mid-2026.
• Sandoz-Amgen Patent Settlement (April 2024): Settlement enables Sandoz to launch denosumab biosimilars Jubbonti and Wyost in the U.S. by May 2025.
• Dr. Reddy’s Collaboration (June 2025): Partnership formed with Alvotech to co-develop a pembrolizumab (Keytruda) biosimilar targeting global markets.
Looking for in-depth insights? Grab the full report: https://www.datamintelligence.com/buy-now-page?report=biosimilars-and-biologics-market
Latest Developments in Biosimilars and Biologics Market
• Interchangeable Approvals: In March 2025, FDA approved Omlyclo (omalizumab biosimilar) as the first interchangeable with Xolair; Bomyntra and Xbryk (denosumab biosimilars) followed suit.
• Aflibercept Biosimilars in US: Multiple biosimilars approved in 2024–25—Yesafili, Pavblu, Ahzantive—targeting eye diseases such as AMD and DME.
• Regulatory Reforms in India: Enhanced quality standards for biosimilar manufacturing are finalizing to position India as a leading global supplier.
Latest News from USA (2025)
In 2025, the FDA approved multiple new interchangeable biosimilars—including omalizumab (Omlyclo) and denosumab variants—marking regulatory momentum toward more flexible substitution at pharmacies. Concurrently, major payers (UnitedHealth, Cigna, CVS) began shifting Humira coverage to favor biosimilars like Amjevita, significantly encouraging usage.
Latest News from Japan (2025)
In January 2025, Biocon Biologics’ ustekinumab biosimilar received PMDA approval in Japan, signaling expanded biologic access and market entry for cost-effective therapies. The launch is expected to influence future biosimilar policy and commercial partnerships in Japan.
Conclusion
According to DataM Intelligence analysis, the biosimilars and biologics market is entering a phase of accelerated global growth. A rapidly expanding pipeline propelled by patent expirations, regulatory clarity, cost pressures, and trust in biosimilar efficacy is redefining the pharmaceutical landscape. With strategic launches, country-specific regulatory reforms, and escalating collaboration, biosimilars are set to democratize access to critical biologic therapies—unleashing affordability without compromising quality.
Unlock 360° Market Intelligence with DataM Subscription Services:
https://www.datamintelligence.com/reports-subscription
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Pipeline Analysis For Drugs Discovery
✅ Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Competitive Landscape
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Related Reports:
Biosimilars market is expected to reach US$ 171.79 billion by 2033
Gluten Intolerance Treatment Market to grow at a CAGR of 11.8% by 2024-2032
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release