COVID-19 Diagnostics Market Analysis 2025–2035: Expected CAGR of 6.5%
COVID-19 diagnostics market grows with govt support, tech advances, at-home testing shift, regulations, and global testing efforts.
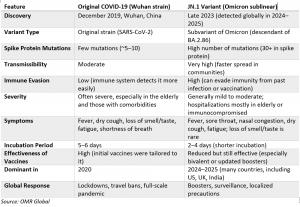
INDORE, INDIA, May 29, 2025 /EINPresswire.com/ -- COVID-19 diagnostics market is anticipated to grow at a CAGR of 6.5% during the forecast period (2025-2035). As the COVID-19 pandemic progresses, additional SARS-CoV-2 virus variations appear, affecting public health and necessitating suitable reactions. JN.1, one of these variations, gained notice owing to its enhanced transmissibility and capacity to partially circumvent immunity. The BA.2.86 variety (sometimes called Pirola) gives rise to the Omicron subvariant JN.1. Furthermore, the emergence of new SARS-CoV-2 variants such as JN.1 highlights the need for sensitive molecular testing platforms, driving market growth and investment in variant-specific assay updates. For instance, in December 2023, HiMedia launched a COVID-19 PCR Kit to detect the JN.1 variant, utilizing the latest genome sequencing data from USAID. The company offers RT-PCR kits for detecting the recently discovered JN.1, SARS-CoV-2 variant, including MBPCR243, MBPCR255, MBPCR262, and MBPCR270. JN.1 is a variant of SARS-CoV-2, causing increasing cases worldwide. The first case was detected in Denmark and later in the USA. Hi-Gx360 tested its Multiplex PCR Kit to detect the JN.1 variant, which has immune-escape capabilities. The company analyzed genome sequencing data from the Global Initiative on Sharing All Influenza Data to determine if their RT-PCR kits can detect the virus.Click To get a Sample PDF (Including Full TOC, Graphs & Charts, Table & Figures) @ https://www.omrglobal.com/request-sample/covid-19-diagnostics-market
Market Trends
Rising demand for rapid and reliable testing kits
The market is expanding owing to the growing demand for swift and dependable testing kits, primarily due to the urgent need for early detection and traceability. According to the National Center for Biotechnology Information, in April 2020, COVID-19 impacted 181 countries, with approximately 1,197,405 confirmed cases. Analyzing daily transmission dynamics and evaluating the effectiveness of control measures is crucial. Data indicates that over 80% of cases are mild, while 14% experience severe complications, and 5% are critical. Current figures show confirmed cases in the US at 308,850, Spain at 126,168, Italy at 124,632, and Germany at 96,092. As of April 4th, 2020, Italy's case fatality rate stands at 13.3%, significantly higher than South Korea's 1.8% and China's 4%. Effective measures include lockdowns in China, Italy, and Spain, the shutdown of nonessential companies in Hubei, combined policies in South Korea, and reduced working hours in Iran.
Emergence of New Variants
The emergence of new SARS-CoV-2 variants necessitates advanced sequencing and multiplex testing platforms for rapid diagnosis and public health strategy. According to the World Health Organization, in March 2025, from January to February 2025, SARS-CoV-2 positive specimens decreased from 7.3% to 5%, with 69,932 weekly tests across 103 countries. The number of new weekly cases globally fell by 16% compared to the previous 28-day period, with over 147,000 cases reported. However, new weekly deaths rose by 28%, totaling over 4,500 fatalities. By February 2025, there were over 777.4 million confirmed cases and more than 7 million deaths globally. Wastewater surveillance suggests that the actual circulation rate is between 2 to 19 times higher than the reported case numbers.
Regional Outlook
Europe to Dominate the COVID Diagnostics Market
Europe led the global COVID diagnostics market with the major market share. This is primarily attributed to the fast growth of the advanced diagnostics and sequencing technologies are crucial for monitoring and tracking the spread of emerging virus variants, especially in Europe with large international travel hubs, driving market growth. According to the World Health Organization, in March 2025, over the past 28 days, Europe recorded 71,219 new COVID-19 cases, which constitutes 48% of the global total for this period, marking a 52% reduction from the previous month. The cumulative case count in Europe has reached 281,105,359, comprising 36% of worldwide cases. During the same timeframe, there were 554 new deaths in Europe, representing 12% of global deaths and showing a 23% decrease from the preceding 28 days. The total death toll in Europe stands at 2,281,070, accounting for 32% of global deaths. Out of 61 countries in the region, 35 (57%) reported new cases, and 14 (23%) reported new deaths in the past month.
Order Your Report Now For A Swift Delivery: https://www.omrglobal.com/buy-now/covid-19-diagnostics-market
The United States has a significant share of the COVID diagnostics market in North America
The growth of the US COVID Diagnostics market is fueled mainly by the pandemic's resurgence and new variants necessitate continuous testing for effective pandemic management and market growth. According to the World Health Organization, in March 2025, in the past 28 days, the Americas reported 69,327 new COVID-19 cases, making up 47% of the global total for that period, and indicating a more than 100% increase from the previous 28-day period. The cumulative confirmed cases in the region have reached 193,404,133, representing 25% of the global total. During the same timeframe, there were 3,990 new deaths reported, accounting for 87% of the global deaths, and showing a 42% rise compared to the prior period. The total COVID-19-related deaths in the Americas now stand at 3,049,466, which is 43% of the global death toll. Additionally, 20 out of 56 countries (approximately 36%) reported new cases, and 6 out of 56 countries (around 11%) reported new deaths.
Market Segmentation and Growth Areas
Reagents & Kits-Based COVID Diagnostics to Become a Larger Segment
The market growth is primarily driven by the increasing demand for robust, multi-target PCR kits due to the emergence of SARS-CoV-2 variants such as Omicron sub-lineages. For December 2024, Thermo Fisher received FDA clearance for its COVID Diagnostic PCR Kit, allowing clinical and public health laboratories to adopt a validated in vitro diagnostic workflow for SARS-CoV-2 detection. The kit's multi-gene target design ensures reliable detection, with real-time results delivered in as fast as three hours. Furthermore, the TaqPath COVID-19 Diagnostic PCR Kit is a real-time PCR solution for detecting SARS-CoV-2 RNA from nasopharyngeal and anterior nasal swabs. It includes assays, controls, and a multi-gene target design for accurate diagnosis. It is suitable for reference/clinical laboratories, public health laboratories, and academic medical centers.
Market Limitations and Challenges
• Declining Testing Demand Post-Pandemic: The demand for sophisticated diagnostic testing is declining as immunization rates increase globally and COVID-19 advances closer to pandemic status in numerous regions. Diagnostic companies' revenues have decreased as a result of this falling need, especially for routine or large amounts of testing, and investor confidence in the market's long-term profitability reduced.
• High Cost of Advanced Diagnostics: Advanced diagnostic tools including genome sequencing, CRISPR-based tests, and RT-PCR are expensive in terms of both infrastructure and running costs. As affordability and availability of healthcare resources continue to be obstacles, this restricts their broad adoption, especially in low- and middle-income nations.
Market Players Outlook
Some of the major players operating in the global COVID Diagnostics market include Abbott Laboratories, Danaher Corp., F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., and Siemens Healthineers AG, among others. Players in the market are highlighting leveraging growth by implementing strategies such as collaboration, partnerships, and expansion in the market, among others. For instance, in December 2024, several initiatives were taken by the Ministry of Electronics and Information Technology (MeitY) to encourage research and innovation in nanoelectronics in India. This depicts government efforts and initiatives to advance the nanomaterial industry.
Recent Developments
• In April 2025, Healthians launched 'Health on Wheels' in Mumbai, a mobile healthcare initiative that provides essential diagnostic services directly to residential communities. The program includes free health screening camps, expert-led wellness consultations, and select blood tests, with discounts on exclusive health packages.
• In March 2023, Kaneka launched a new PCR test kit, the KANEKA RT-PCR Kit SARS-CoV-2 (Omicron/Delta) ver.2, that can detect the Omicron (BA.1), "Stealth" Omicron (BA.2), and Delta variants of COVID-19 simultaneously. The kit uses a proprietary reagent developed using Kaneka's molecular testing-related technologies, reducing the testing burden and aiding in selecting suitable drugs and treatment approaches. The kit is available for purchase at a suggested retail price of 121,000 yen.
• In May 2023, Creative Diagnostics introduced new SARS-CoV-2 immunoassay kits for research, including IgG, IgM, Antigen, and Total Antibody ELISA kits for direct detection of the new coronavirus.
• In April 2020, Genalyte launched its SARS-CoV-2 Multi-Antigen Serology Panel, a rapid COVID-19 diagnostic that tests for IgG and IgM antibodies produced by the body in response to the virus. The panel, run on Genalyte's Maverick platform, achieves 100% specificity on 300 negative patients. The test is available as a Laboratory Developed Test, with Genalyte aiming to test over 7,500,000 patients per month by September.
Conclusion
The emergence of the JN.1 COVID-19 variant has presented challenges across various industries. Healthcare systems are dealing with increased risks to vulnerable groups, while travel and tourism are coping with renewed restrictions. The insurance and fintech sectors are adjusting to rising demand and associated risks, and the real estate market is moving towards safer, remote-friendly environments. Workplaces are implementing enhanced safety protocols, and the global COVID-19 diagnostics market is experiencing a rise in demand for accurate, variant-compatible testing, prompting companies to update RT-PCR and rapid antigen kits. Despite facing regulatory and financial challenges, innovation and adaptability continue to be essential in managing the ongoing impacts of the pandemic.
Request for Customization: https://www.omrglobal.com/report-customization/covid-19-diagnostics-market
Frequently Asked Questions (FAQs)
1. How does JN.1 differ from other variants?
JN.1 has mutations that increase transmissibility and immunity evasion, but it doesn't seem to cause more severe disease than previous variants.
2. Are the current COVID-19 tests effective in detecting JN.1?
Yes, Standard PCR and rapid antigen tests can detect JN.1 infections, but specific variant identification requires genomic sequencing, typically used for surveillance purposes.
3. Is JN.1 Already in India?
JN.1 is already circulating in India, with subvariants slightly different from Hong Kong and China. Despite being highly infectious, it typically causes mild illness without new alarming clinical patterns.
4. Should I be concerned about the rise in cases due to JN.1?
Maintaining preventive measures and staying up-to-date with vaccinations can significantly reduce the risk of severe illness due to the increased transmissibility of JN.1.
Some of the Key Companies in the COVID Diagnostics Market Include-
• Abbott Laboratories
• Abingdon Health Group
• Becton, Dickinson and Co. (BD)
• bioMérieux SA
• Bio-Rad Laboratories, Inc.
• Cepheid
• Danaher Corp.
• DiaSorin S.p.A.
• Eurofins Scientific
• F. Hoffmann-La Roche Ltd.
• Hologic Inc.
• Hotgen Biotech Co., Ltd.
• Illumina Inc
• Innova Medical Group Inc.
• Myriad Genetics, Inc.
• OraSure Technologies Inc.
• OraSure Technologies, Inc.
• PerkinElmer Inc.
• Quest Diagnostics Inc.
• Quidel Corp.
• Seegene Inc.
• Siemens Healthineers AG
• SYNLAB International
• Thermo Fisher Scientific Inc.
COVID Diagnostics Market Segmentation Analysis
Global COVID Diagnostics Market by Type
• Instruments
• Reagents & Kits
Global COVID Diagnostics Market by Test Type
• Molecular Diagnostics
• Serology / Antibody Tests
• Antigen Tests
• Others (Next Generation Sequencing (NGS), and Biosensors and Point-of-Care Devices)
Global COVID Diagnostics Market by Sample Type
• Oropharyngeal & Nasopharyngeal Swabs
• Blood
• Urine
• Saliva
• Other
Global COVID Diagnostics Market by End User
• Hospitals & Clinics
• Diagnostic Laboratories
• Research Institutes
• Point-of-Care Settings
• Home Testing / Self-Testing
Regional Analysis
• North America
o United States
o Canada
• Europe
o UK
o Germany
o Italy
o Spain
o France
o Rest of Europe
• Asia-Pacific
o China
o India
o Japan
o South Korea
o ASEAN Economies (Singapore, Thailand, Vietnam, Indonesia, and Other)
o Australia and New Zealand
o Rest of Asia-Pacific
• Rest of the World
o Latin America
o Middle East and Africa
Inquiry Before Buying: https://www.omrglobal.com/inquiry-before-buying/covid-19-diagnostics-market
Anurag Tiwari
Orion Market Research Pvt Ltd
+ +91 91798 28694
email us here
Visit us on social media:
LinkedIn
Facebook
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release